The State of US Vaccine Policy — Feb 19, 2026
Federal courts, state legislatures, and a public health system under strain
Here we are—the second biweekly installment of The State of US Vaccine Policy, our ongoing series in partnership with CIDRAP (the Center for Infectious Disease Research and Policy at the University of Minnesota). The PDF version is here.
Two weeks ago, we were humbled by the conversations that followed our first issue. So many people are out there tracking these policies and their implications in depth—researchers, advocates, clinicians—and we’re blown away by what we’re finding. The public health response to what’s unfolding has been remarkable: states moving fast, medical organizations holding the line, legal teams working around the clock, communicators finding creative ways to reach people. We’ll be linking to as much of it as we can throughout this piece, because part of what we want this series to be is a jumping-off point, connecting you to the people on the ground doing this work and the resources they are creating.
But before we dive in, we think it’s important to acknowledge that while this is a briefing about vaccine policy, it’s really about our kids, our neighbors, our most vulnerable community members—all of us. It’s about whether the systems we built and refined over decades to protect people from preventable diseases are still standing, and who’s still fighting to keep them there.
So wherever you’re coming from right now, we see you:
- If you’re a busy parent who just wants to know what’s happening but has zero bandwidth for action steps right now, that’s okay. You showed up, and that matters.
- If you’re a 5 Calls regular looking for new material—we’ve got you!
- And if you’re a clinician, a researcher, or an advocate trying to stay oriented in a very fast-moving moment—welcome, we’re glad you’re here, and we hope this helps.
Between federal lawsuits, sweeping schedule changes, and states mobilizing their own legal challenges, the last few weeks have been a whirlwind—and things are only picking up speed. Let’s discuss…
The short version: this lawsuit is moving fast, it’s growing, and a ruling could come any day now. Regardless (and breaking just today), the ACIP meeting previously scheduled for next week will be delayed.
As a refresher from a couple weeks ago: the American Academy of Pediatrics (AAP) and other medical organizations filed a lawsuit in July 2025 against Health Secretary Robert F. Kennedy Jr. and others at the Department of Health and Human Services (HHS), alleging that removing the COVID-19 vaccine from the recommended schedule for children and pregnant women was done without following the proper procedures required by federal law.
Over six months later, the case has grown substantially. Four amendments have been made to the original complaint, each expanding its scope. It now includes challenges to RFK Jr.’s removal of COVID-19 vaccination recommendations for both children and adults, and his decision to fire and replace all 17 members of the Advisory Committee on Immunization Practices (ACIP). The most recent amendment, filed on January 19th, responds to the sweeping childhood vaccine schedule changes announced by HHS leadership on January 5th—again, bypassing the scientific process entirely.
February 9th was a busy day in the courtroom. Most significantly, the AAP filed a motion seeking a preliminary injunction. Think of it like a baby gate at the top of the stairs—it’s not a permanent fix, but it’s a way to prevent serious harm while you figure out what to do next. In legal terms, it’s a temporary court order that would block the new childhood vaccine schedule changes from being enforced, and would also stop the ACIP meeting from proceeding—a meeting that has since been delayed to mid-March, regardless—until the court can determine whether the reconstituted committee’s formation was even lawful in the first place.
Three days later, the AAP filed additional sworn declarations from experts and medical leaders, adding fresh evidence of harm in direct response to arguments the government raised. Both sides presented their arguments before a judge on Friday, February 13th. After a full day in court, the judge did not rule from the bench. At the time of this writing, we are still waiting.
- In a late-breaking development yesterday, Children’s Health Defense (CHD)—the organization RFK Jr. co-founded—filed a motion to intervene in the case today. The AAP’s legal team was direct in response: CHD waited over six months after the initial complaint was filed and moved only now, right before the judge is expected to rule. They say the intervention is without merit and will be vigorously opposed.
- Meanwhile, the ACIP meeting may be postponed anyway, and for an entirely different reason than the injunction. HHS has missed both the seven-day and fifteen-day Federal Register notice deadlines legally required before an advisory committee meeting. As of yesterday, no notice has been posted. CDC staff were told at an all-hands meeting on Tuesday that the meeting likely will not take place as scheduled. In the same meeting, a communications officer told staff that NCIRD—the CDC center that has historically supported ACIP—no longer “owns” the committee. In the past hour, that’s been confirmed: the ACIP meeting has officially been delayed to mid-March, though no date has been set.
While things plays out in federal court, California Attorney General Rob Bonta announced Tuesday that his office is preparing its own lawsuit challenging the federal pediatric vaccine schedule changes. Connecticut Attorney General William Tong has confirmed that his office is working with California, signaling that this could become a multistate filing. This is all in the early stages for now, but the fact that the country’s most heavily populated state is getting involved adds significant political weight to the growing pushback against the administration’s vaccine policies.
It’s worth stepping back to see the fuller picture of what CHD is doing legally. On the same day they filed the motion to intervene in AAP v. Kennedy, they are also—separately—suing the AAP itself. Earlier this year, CHD filed a federal civil RICO lawsuit against the Academy. RICO (the Racketeer Influenced and Corrupt Organizations Act) is a law originally designed to prosecute organized crime. Applying it here means CHD is arguing that decades of mainstream vaccine guidance amounted to a coordinated criminal conspiracy to defraud families, targeting the AAP alongside vaccine manufacturers for allegedly concealing gaps in safety testing.
Legal experts note that civil RICO cases like this frequently fail at the pleading stage— converting scientific and policy disagreements into racketeering claims faces significant legal hurdles. But that may not be the point. The strategy appears designed to pressure medical organizations into a defensive posture, drain resources, and signal to other professional societies that defending evidence-based medicine comes with a cost. The AAP is now fighting on multiple fronts simultaneously: challenging the dismantling of vaccine policy in federal court, opposing CHD’s attempt to insert itself into that same case, and now defending the very act of recommending vaccines in the first place.
In the middle of all of this, the American College of Obstetricians and Gynecologists (ACOG) released updated guidance on Tuesday, reaffirming the importance of vaccines during pregnancy. The guidance recommends that OB-GYNs routinely assess patients’ vaccination status and recommend needed vaccines, explicitly addressing flu, COVID-19, RSV, and Tdap. It also directly addresses misinformation as a factor in eroding vaccine confidence, noting that changing national recommendations are creating confusion for both patients and clinicians. The timing is notable given that two of the newly appointed ACIP members are OB-GYNs whose history of vaccine skepticism has prompted calls for removal by dozens of Democratic lawmakers. ACOG is making clear where the evidence-based obstetric community stands.
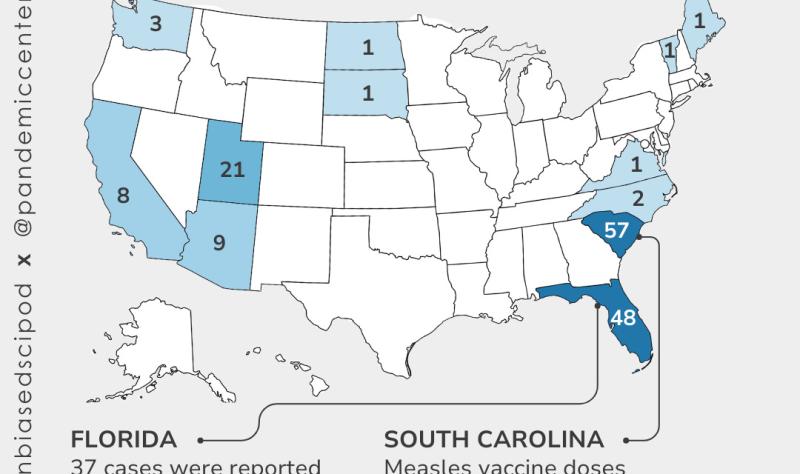
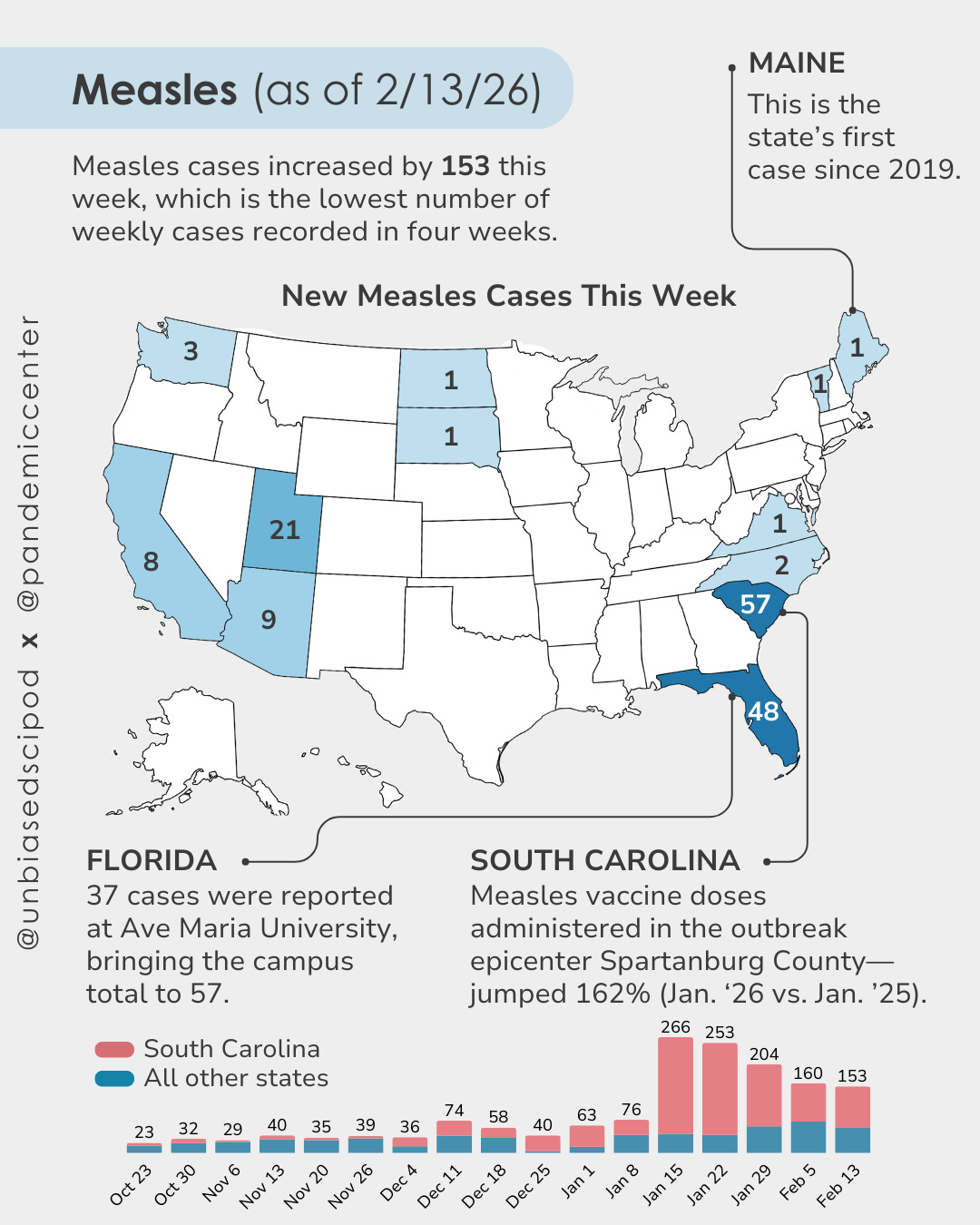
Reminder: school vaccine requirements are one of the most effective tools we have for maintaining the coverage levels needed to prevent outbreaks. When those requirements go away, vaccination rates drop, and diseases come back. We’re already seeing it: a measles outbreak in South Carolina has sickened nearly 1,000 people, with at least 20 hospitalized.
As we mentioned two weeks ago, groups like the Medical Freedom Act Coalition are actively pushing to overturn state-level vaccine requirements across the country, and they are piggybacking on the recent federal vaccine schedule changes as fuel. Most state bills haven’t seen major movement since our last issue (which you can read here). New Hampshire and New York are the notable exceptions.
On February 12th, the New Hampshire House narrowly passed a bill that would remove hepatitis B from the list of vaccines required for children to attend school or daycare. Supporters framed the bill as an effort to align state and federal government recommendations. The bill still has several steps before it becomes law—it goes to the House Finance Committee next, and then has to pass the full Senate. It’s worth noting that New Hampshire’s governor vetoed a similar bill last year.
Oregon offers a window into what’s already happening on the ground, even before new legislation passes. State health officials there have tracked a drop in newborn hepatitis B vaccination over the past four years, from 86% of infants receiving the birth dose in 2022 to 82% in 2024, with some counties as low as 60%. Prenatal screening rates are falling alongside it. This matters because up to 90% of infants infected with hepatitis B at birth develop chronic liver infections, and without treatment, a significant portion will eventually die from cirrhosis or liver cancer. The CDC stopped recommending routine newborn hepatitis B vaccination in December, a move ignored by the AAP and most states but already creating confusion on the ground. Oregon’s data are a preview of what sliding coverage looks like in practice, before an outbreak forces anyone to pay attention.
In New York, anti-vaccine groups are mobilizing against bills that would allow the state Health Commissioner to follow vaccine recommendations from professional organizations like the AAP rather than solely relying on federal ACIP guidance. The Autism Action Network, led by an influential anti-vaccine activist John Gilmore, is coordinating opposition by claiming these bills represent a “power grab”, arguing that vaccines that are not ACIP-approved would lose manufacturer liability protections and federal Vaccines for Children (VFC) funding. This represents a tactical shift where anti-vaccine activists are using arguments about federal funding and legal immunity to mobilize their base against pro-public health legislation.
This one moved fast, so buckle up. Last week, top U.S. Food and Drug Administration (FDA) official Vinay Prasad rejected Moderna’s application to even submit its mRNA flu vaccine for review on the basis that the study was not “adequate or well-controlled” because it used a control arm that the FDA felt did not represent the “best-available standard of care.” This was despite Phase 3 trial data showing the vaccine was safe and effective, and an overruling of career scientists. The rejection sparked significant industry backlash and raised serious questions about the FDA’s decision-making under the current administration.

Fast forward to yesterday: the FDA reversed course, and it will now review Moderna’s application after all. If approved, the vaccine will be available to adults 50 and older for the 2026-2027 flu season. As for why this happened, according to Politico, President Trump called FDA Commissioner Marty Makary to the White House to express frustration over the agency’s handling of vaccine issues, and the Type A meeting with Moderna that followed was scheduled more quickly than is typical. One source described it as giving the agency “a public way to save face.” The White House has denied that Trump influenced the reversal. Whatever the explanation, the whiplash has already done damage; vaccine manufacturers are watching closely, with some reconsidering their U.S. development plans entirely, which is bad news not just for innovation but for pandemic preparedness.
A couple of related notes worth flagging: despite formally leaving the World Health Organization (WHO) at the end of January, the U.S. is still participating in the WHO’s upcoming meeting to discuss which flu strains will go into next year’s vaccine. And the FDA has opened a public docket ahead of a March 12th meeting on the same topic—public comments are open until March 3rd if you’d like to weigh in.
Announced late on Wednesday, Dr. Jay Bhattacharya, who currently serves as director of the National Institutes of Health, will also take on the role of acting CDC director—serving both roles simultaneously. He replaces Jim O’Neill, who had been serving as acting CDC director since last August, when RFK Jr. forced out Senate-confirmed director Susan Monarez after less than a month on the job. Dr. Bhattacharya is best known as a co-author of the Great Barrington Declaration, which during the COVID-19 pandemic advocated allowing the virus to spread freely among healthy populations while directing resources toward the elderly and vulnerable—a position that drew sharp criticism from public health leaders.
The information infrastructures we’ve relied on to understand vaccine safety, disease trends, and outbreak risk are being quietly hollowed out. Dozens of CDC databases that public health officials, researchers, and clinicians depend on for real-time decision-making have gone dark, and with little explanation. When those data streams disappear, it does more than just create a knowledge gap. It makes it harder for everyone—from your child’s doctor to your state and local health department—to give you accurate and timely information on what diseases are actually circulating in your community. And it erodes the kind of public trust that, once lost, is very difficult to rebuild.
This is exactly the gap that Brown University’s Pandemic Center has stepped in to fill. Their team has been pulling together data systems and publishing weekly Tracking Reports to help make sense of the disease landscape, even when the CDC goes quiet. If you want to stay informed beyond the headlines, we’d encourage you to subscribe. Unsure of what the declines in childhood vaccinations really mean? Researchers at Emory University have created the VaxImpactMap, an interactive tool that lets you explore the consequences of reduced vaccine coverage for rotavirus, pertussis, and pneumococcal diseases. We threw a lot at you today. If you’re sitting there scratching your head and trying to understand how this all fits together, our colleagues at the Common Health Coalition have put together this infographic to outline the short-, medium-, and long-term impacts of these changes to the immunization landscape.
A note before you go: by the time you read this, something in it will likely have moved. We rewrote sections of this briefing a dozen times in the last 48 hours alone—a CHD motion filed, an ACIP meeting thrown into question, a new CDC director named, a lawsuit amended. That’s not a complaint. It’s just the reality of this moment, and it’s part of why we think a resource like this matters. We can’t promise we’ll catch everything, but we can promise we’ll keep showing up, keep updating, and keep trying to make sense of it alongside you.
And lastly, this is only our second issue. We’re still shaping what it can be. While exciting collaborations are in the works, we want to hear from you—our readers, our caretakers, our busy parents. How can we help you feel empowered to stay in the loop? Leave us a note in the comments.
Stay curious,
Unbiased Science
This update is also posted on Unbiased Science’s Substack page.
To receive these updates on the day they’re published, subscribe to CIDRAP’s free Daily News Headlines newsletter.
First Appeared on
Source link






