Scientists are racing to grow human teeth in the lab
It’s not surprising that many people fear the dentist. Replacing a tooth often requires invasive surgery and implanting a titanium screw into a patient’s jawbone, then waiting months for that to strengthen into an artificial root, before attaching a crown or cap on top of it.
But research groups around the world are working to find ways to implant or grow real biological teeth in a human jaw.
That may be a way off, but at King’s College London, Ana Angelova Volponi, director of the postgraduate program in regenerative dentistry, has been experimenting with lab-grown teeth for almost two decades, and was part of a team that in 2013 grew a tooth from human and mouse cells.
This year, she led a study that built upon that work and achieved a breakthrough in the material used to house the growing tooth in the lab, which better mimics the actual environment where biological teeth grow in the mouth. It’s a key step on the way to replacing the mouse cells with human cells and stimulating them to form a tooth.
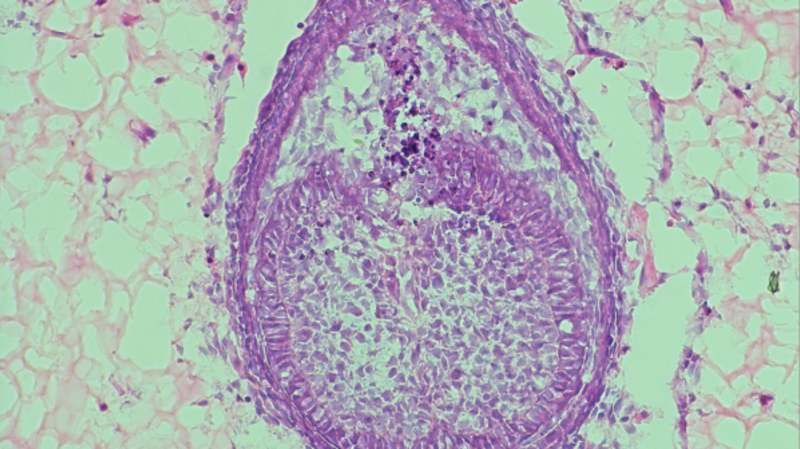
The idea of creating lab-grown teeth dates back to the 1980s, Volponi said, but the one that she and her colleagues created over a decade ago was the first that used adult human gingival cells — which make up the gums and are obtained by lightly scratching the inside of the mouth — and combined them with “progenitor” tooth cells taken from a mouse embryo.
“It’s almost like a tripod,” she said about the elements that contribute to growing a tooth in a lab setting. “The two types of cells are engaging in tooth making, in a sort of conversation, and then we have the environment where this happens.”
The environment, which researchers call a “scaffold,” is essential to the formation of the lab-grown tooth, and it’s the subject of Volponi’s most recent study. In 2013, Volponi used a scaffold made of the protein collagen, but now uses a hydrogel, a type of polymer with a high water content, explained Xuechen Zhang, a doctoral student at King’s College London, and a co-author of the study. “We gather the cells first from the mouse embryos and then mix them together and spin them down to get a small cell pellet,” he said. “Then we inject this cell pellet inside the hydrogel and grow it for around eight days.” Because the work focused on the environment, human cells aren’t needed.
At the end of the eight days, tooth-like structures will have formed inside the hydrogel, which was developed in collaboration with Imperial College London. In the 2013 research, these “tooth primordia” were transferred into a mouse where they developed into a tooth structure complete with developing roots and enamel.
Many challenges remain before a lab-grown tooth can be used in a human patient, but the new material helps with some pieces of that puzzle, Volponi said, by improving the “conversation” between the cells that are tasked with making a tooth.
Researchers still don’t know exactly how to replace the embryonic mouse cells with adult human cells, but if that puzzle is solved, Volponi envisions two possible ways to integrate lab-grown teeth into everyday dentistry: “We either grow a tooth up to a certain stage of development, and then embed it into the (tooth socket), where a lost tooth was and where the new one will have the potential to fully grow into a biological tooth, incorporating itself within the organic structures such as the bone and the ligament. Or, we fully grow the tooth first and then implant it surgically. It’s still too early to say which approach will be more viable.”
A real, biological replacement tooth grown from a patient’s own cells would offer many advantages over a crown or implant. First, it would be accepted into the tissue without inflammation or rejection, but it would also feel exactly like a real tooth — unlike implants that lack feeling and elasticity as they are simply fused into the bone.
According to Vitor C. M. Neves, a senior clinical lecturer at the School of Clinical Dentistry of the University of Sheffield in England, Volponi has long been a pioneer in the field of whole tooth regeneration, serving as an inspiration to many researchers around the world. “Her new research tackles a key factor in the production and potential industrialization of this technology — the use of matrices in whole tooth regeneration,” said Neves, who was not involved with Volponi’s study.
The findings, he added, highlight the importance of creating an environment that can support whole tooth engineering for clinical application: “The more researchers who contribute to advancing this field, the sooner humanity will be able to reap its benefits.”
Other researchers working in the same field are using a variety of different techniques to grow teeth.
Katsu Takahashi and his colleagues at the Medical Research Institute Kitano Hospital in Osaka are developing an antibody-based treatment aimed at promoting the growth of teeth in people with conditions such as anodontia, or the congenital lack of teeth. The treatment has entered human clinical trials and could be ready by the end of the decade.
In late 2024, a team led by Pamela Yelick at the School of Dental Medicine of Tufts University, grew human-like teeth — created from human and pig cells — in pigs. Pigs, unlike humans, regrow their teeth several times over the course of their lives. The ultimate aim is to prompt cells in a human jaw to grow new teeth, without using any pig cells.
At the University of Washington, a team led by Hannele Ruohola-Baker, a professor of biochemistry and an associate director of the university’s Institute for Stem Cell and Regenerative Medicine, has successfully grown dental pulp stem cells from human stem cells mined from donated wisdom teeth: “We aim to uncover the molecular blueprint of human tooth formation and to recreate that process in the laboratory,” she said. “While Volponi’s study builds tooth-like structures from existing dental tissues, our platform generates the key human tooth-forming cell types (from scratch) and guides them along authentic developmental trajectories.”
As for when the fruits of all this research will become available, Ruohola-Baker believes we won’t have to wait that long. “Although clinical translation will take time, momentum in this field is accelerating, heralding a future in which biological tooth repair or replacement becomes a realistic option within the coming decade,” she said.
First Appeared on
Source link






