
Natural Compound in Dark Chocolate and Coffee Linked to Slow Aging in Major DNA-Based Human Study
A key ingredient in dark chocolate has emerged as a potential ally in the biology of aging. Backed by new molecular evidence from large-scale population studies, the compound already consumed worldwide in cocoa and coffee has shown consistent links to slower cellular aging.
The research, published in late 2025, identifies theobromine, a naturally occurring plant alkaloid, as a standout molecule associated with longer telomeres and reduced DNA-based signs of age progression. The findings, drawn from blood samples and DNA methylation data in over 1,600 individuals, point to a measurable connection between a common dietary compound and the pace of human biological aging.
Rather than promote a miracle food, researchers mapped molecular patterns across two well-established European cohorts and uncovered one of the strongest statistical links to date between diet and aging biology. Theobromine, long overshadowed by caffeine, is now the subject of renewed scientific interest.
What distinguishes this work is not what participants ate, but what was found in their blood.
Slower Aging Traced Through Methylation and Telomere Patterns
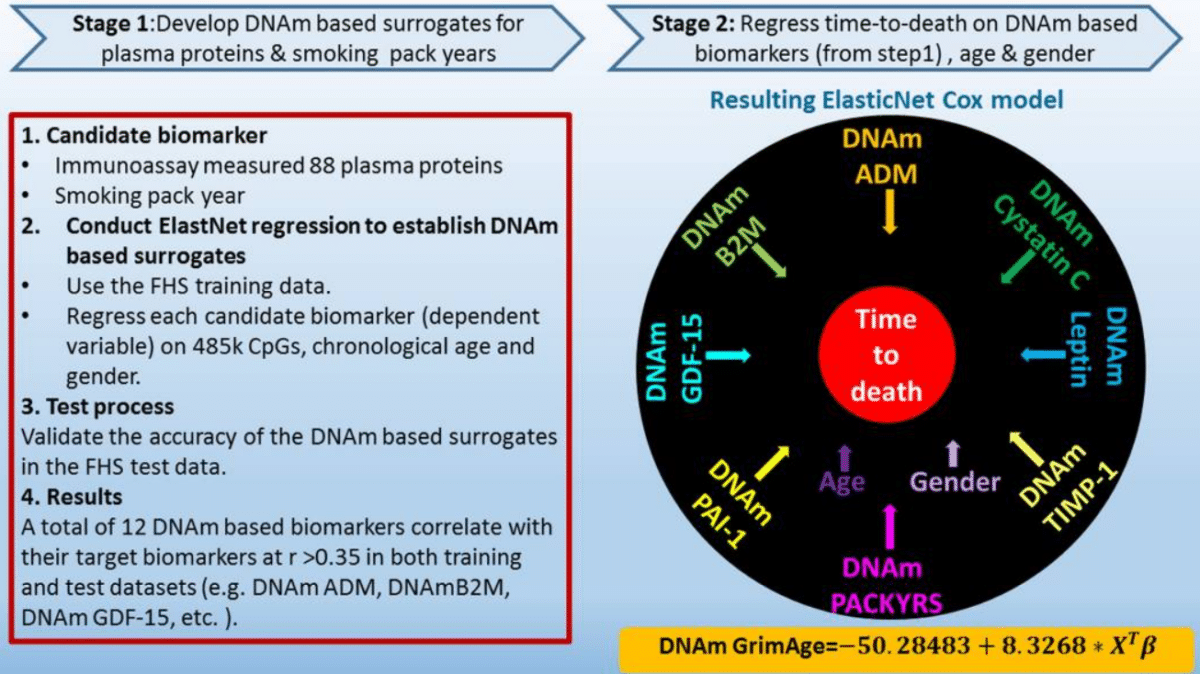
Research teams from King’s College London and Helmholtz Zentrum München investigated six methylxanthine compounds commonly found in chocolate and coffee. One stood out. Theobromine was consistently associated with reduced epigenetic age acceleration and longer telomere estimates.
In the TwinsUK cohort, which included 509 adult female participants, elevated blood levels of theobromine were linked to lower scores on GrimAge, a DNA methylation clock known for predicting mortality risk. Participants also showed higher values of DNAmTL, a methylation-based proxy for telomere length, often used as a biomarker of cellular aging.
Validation came from the KORA cohort in Germany, where 1,160 adults were analyzed. In both populations, higher levels of circulating theobromine aligned with molecular signatures indicating slower biological aging.
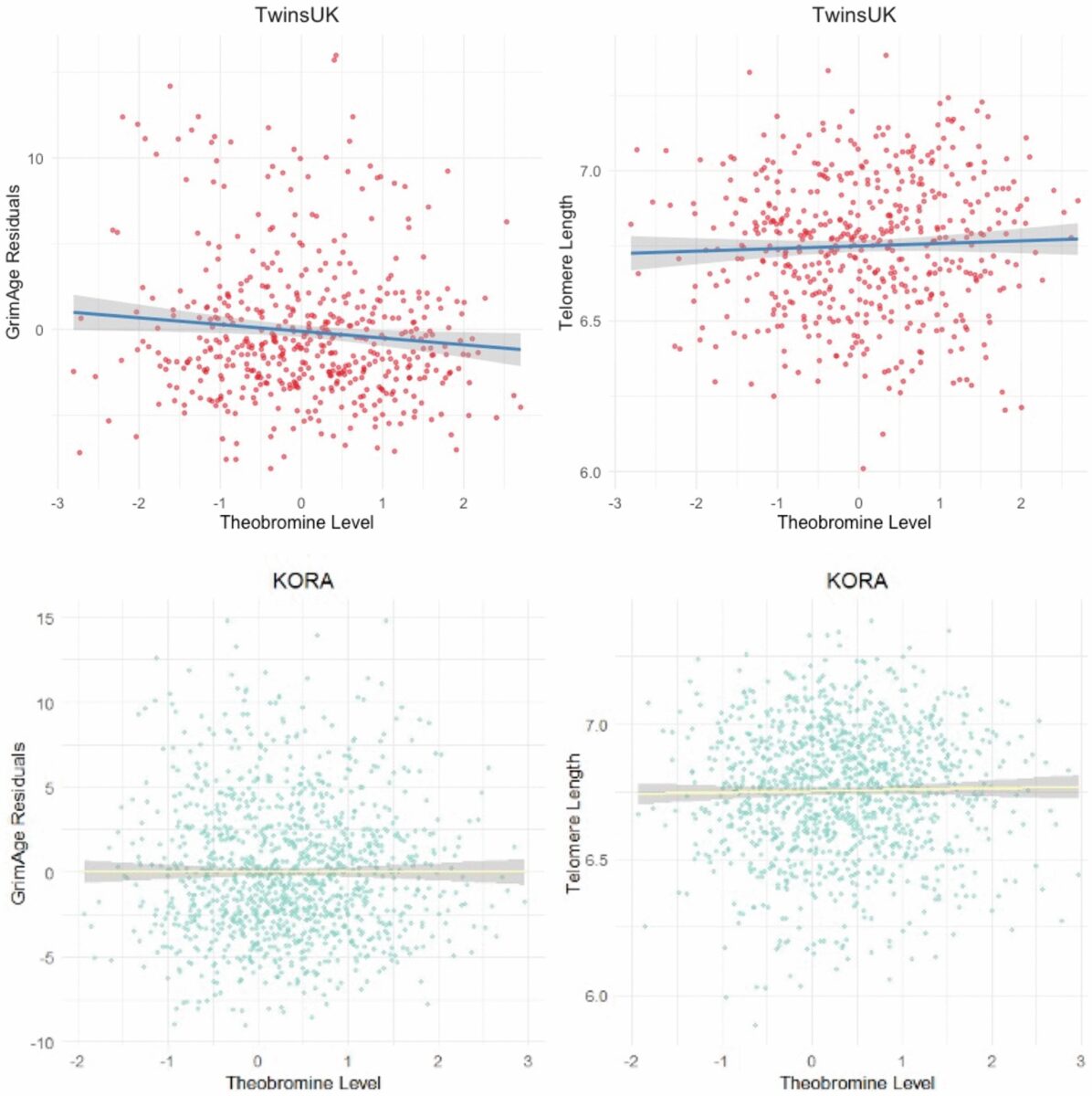
Statistical models controlled for potential confounders, including age, body mass index, smoking history, and levels of other methylxanthines such as caffeine, paraxanthine, and theophylline. Even after accounting for these variables, theobromine remained the strongest predictor of reduced biological aging across both epigenetic clocks.
Feature selection techniques including LASSO regression and elastic-net modeling confirmed the robustness of theobromine’s association, ruling out multicollinearity from related metabolites.
A Common Molecule With Unexpected Effects
Theobromine is widely consumed through dark chocolate, cocoa powder, and coffee, although it is often overlooked in favor of its stimulant cousin caffeine. It is less potent neurologically but has been previously linked to vascular health, cholesterol modulation, and anti-inflammatory activity in smaller studies.
Now, findings published in Aging offer a new perspective. Using mass spectrometry and genome-wide methylation profiling, researchers found that theobromine levels in the blood correlated strongly with biological age indicators. These links were particularly robust when blood sampling for metabolomic and epigenetic measurements occurred within a close time window.
Participants with recent exposure to theobromine tended to exhibit DNA methylation patterns typically seen in younger individuals. In a notable finding, the relationship between theobromine and slower aging was most pronounced in former smokers, suggesting potential interaction with cellular repair mechanisms related to oxidative stress or DNA damage.
Still No Proof of Causality
Despite strong associations, this was an observational study. Researchers emphasized that the findings do not prove theobromine slows aging, only that it is statistically linked with molecular signatures of slower aging in two independent populations.
The study also explored whether theobromine could be acting in synergy with other bioactive compounds. Cocoa is rich in polyphenols and flavan-3-ols, which have previously shown benefits for blood vessel function and inflammation. A 2023 meta-analysis cited in a related review noted that methylxanthines may enhance the activity of flavanols but are unlikely to produce similar effects on their own.
Theobromine levels were only weakly correlated with self-reported chocolate consumption and were not strongly associated with overall diet quality, suggesting that metabolic differences, gut microbiome variation, or genetic factors may contribute to its presence in circulation.
Moreover, evidence from a previous study indicates that telomere length and methylation-based clocks reflect distinct aspects of aging biology. Theobromine’s simultaneous association with both supports the idea that it may influence multiple aging pathways, although the mechanisms remain unclear.
A Future Target for Intervention
The results suggest that common dietary exposures may have a measurable impact on biological aging, particularly at the level of the epigenome. Theobromine now joins a shortlist of compounds, along with resveratrol and flavonoids, that are attracting serious interest in the context of longevity research.
Still, researchers stopped short of recommending dietary changes or supplements. Controlled trials will be needed to establish whether increasing theobromine intake can meaningfully influence epigenetic age or telomere maintenance. Future studies may also examine how the compound behaves across different populations, age groups, and genetic profiles.
First Appeared on
Source link






