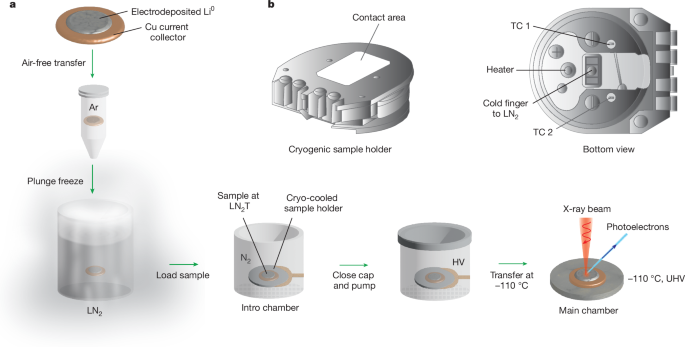
Cryogenic X-ray photoelectron spectroscopy for battery interfaces
Oyakhire, S. T., Gong, H., Cui, Y., Bao, Z. & Bent, S. F. An X-ray photoelectron spectroscopy primer for solid electrolyte interphase characterization in lithium metal anodes. ACS Energy Lett. 7, 2540–2546 (2022).
Yu, W., Yu, Z., Cui, Y. & Bao, Z. Degradation and speciation of Li salts during XPS analysis for battery research. ACS Energy Lett. 7, 3270–3275 (2022).
Bard, A. J. et al. ChemInform Abstract: the electrode/electrolyte interface – a status report. J. Phys. Chem. 97, 7147–7173 (1993).
Yu, X. & Manthiram, A. Electrode-electrolyte interfaces in lithium-based batteries. Energy Environ. Sci. 11, 527–543 (2018).
Stamenkovic, V. R., Strmcnik, D., Lopes, P. P. & Markovic, N. M. Energy and fuels from electrochemical interfaces. Nat. Mater. 16, 57–69 (2017).
Lin, D., Liu, Y. & Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 12, 194–206 (2017).
Li, Y., Leung, K. & Qi, Y. Computational exploration of the Li-electrode|electrolyte interface in the presence of a nanometer thick solid-electrolyte interphase layer. Acc. Chem. Res. 49, 2363–2370 (2016).
Winter, M. The solid electrolyte interphase – the most important and the least understood solid electrolyte in rechargeable Li batteries. Z. Phys. Chem. 223, 1395–1406 (2009).
Dedryvère, R. et al. XPS identification of the organic and inorganic components of the electrode/electrolyte interface formed on a metallic cathode. J. Electrochem. Soc. 152, A689 (2005).
Kanamura, K., Tamura, H., Shiraishi, S. & Takehara, Z.-I. XPS analysis for the lithium surface immersed in γ-butyrolactone containing various salts. J. Electrochem. Soc. 40, 913–921 (1995).
Andersson, A. M. & Edström, K. Chemical composition and morphology of the elevated temperature SEI on graphite. J. Electrochem. Soc. 148, A1100 (2001).
Li, Y. et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo-electron microscopy. Science 358, 506–510 (2017).
Boyle, D. T. et al. Corrosion of lithium metal anodes during calendar ageing and its microscopic origins. Nat. Energy 6, 487–494 (2021).
Steinrück, H. G. et al. Interfacial speciation determines interfacial chemistry: X-ray-induced lithium fluoride formation from water-in-salt electrolytes on solid surfaces. Angew. Chem. Int. Ed. 59, 23180–23187 (2020).
Zhang, Z. et al. Capturing the swelling of solid-electrolyte interphase in lithium metal batteries. Science 375, 66–70 (2022).
Wang, X. et al. New insights on the structure of electrochemically deposited lithium metal and its solid electrolyte interphases via cryogenic TEM. Nano Lett. 17, 7606–7612 (2017).
Zachman, M. J., Tu, Z., Choudhury, S., Archer, L. A. & Kourkoutis, L. F. Cryo-STEM mapping of solid–liquid interfaces and dendrites in lithium-metal batteries. Nature 560, 345–349 (2018).
Zhang, Z. et al. Cryogenic electron microscopy for energy materials. Acc. Chem. Res. 54, 3505–3517 (2021).
Bai, X., McMullan, G. & Scheres, S. H. W. How cryo-EM is revolutionizing structural biology. Trends Biochem. Sci 40, 49–57 (2015).
Dubochet, J. On the development of electron cryo-microscopy (Nobel Lecture). Angew. Chem. Int. Ed. 130, 10842–10846 (2018).
Henderson, R. From electron crystallography to single particle CryoEM (Nobel Lecture). Angew. Chem. Int. Ed. 130, 10804–10825 (2018).
Taylor, K. A. & Glaeser, R. M. Electron diffraction of frozen, hydrated protein crystals. Science 186, 1036–1037 (1974).
McDowall, A. W. et al. Electron microscopy of frozen hydrated sections of vitreous ice and vitrified biological samples. J. Microsc. 131, 1–9 (1983).
Henderson, R., Baldwin, J. M., Downing, K. H., Lepault, J. & Zemlin, F. Structure of purple membrane from Halobacterium halobium: recording, measurement and evaluation of electron micrographs at 3.5 Å resolution. Ultramicroscopy 19, 147–178 (1986).
Fernandez-Moran, H. Cell-membrane ultrastructure: low-temperature electron microscopy and X-ray diffraction studies of lipoprotein components in lamellar systems. Circulation 26, 1039–1065 (1962).
Tan, J., Matz, J., Dong, P., Shen, J. & Ye, M. A. A growing appreciation for the role of LiF in the solid electrolyte interphase. Adv. Energy Mater. 11, 2100046 (2021).
Li, T., Zhang, X.-Q., Shi, P. & Zhang, Q. Fluorinated solid-electrolyte interphase in high-voltage lithium metal batteries. Joule 3, 2647–2661 (2019).
Wang, C., Meng, Y. S. & Xu, K. Perspective—fluorinating Interphases. J. Electrochem. Soc. 166, A5184–A5186 (2019).
Hobold, G. M., Wang, C., Steinberg, K., Li, Y. & Gallant, B. M. High lithium oxide prevalence in the lithium solid–electrolyte interphase for high Coulombic efficiency. Nat. Energy 9, 580–591 (2024).
Kim, M. S. et al. Suspension electrolyte with modified Li+ solvation environment for lithium metal batteries. Nat. Mater. 21, 445–454 (2022).
Lin, D. et al. Fast galvanic lithium corrosion involving a Kirkendall-type mechanism. Nat. Chem. 11, 382–389 (2019).
Arkel, A. E., Spitsbergen, U. & Heyding, R. D. Note on the volatility of lithium oxide. Can. J. Chem. 33, 446–447 (1955).
Kudo, H., Wu, C. H. & Ihle, H. R. Mass-spectrometric study of the vaporization of Li2O(s) and thermochemistry of gaseous LiO, Li2O, Li3O, and Li2O2. J. Nucl. Mater. 78, 380–389 (1978).
Seah, M. P. & Dench, W. A. Quantitative electron spectroscopy of surfaces: a standard data base for electron inelastic mean free paths in solids. Surf. Interface Anal. 1, 2–11 (1979).
D’Acunto, G. et al. Atomic layer deposition of hafnium oxide on InAs: insight from time-resolved in situ studies. ACS Appl. Electron Mater 2, 3915–3922 (2020).
Walther, T. in Microscopy Methods in Nanomaterials Characterization 105–134 (Elsevier, 2017).
García De Abajo, F. J. & Di Giulio, V. Optical excitations with electron beams: challenges and opportunities. ACS Photon. 8, 945–974 (2021).
Jagger, B. & Pasta, M. Solid electrolyte interphases in lithium metal batteries. Joule 7, 2228–2244 (2023).
He, M., Guo, R., Hobold, G. M., Gao, H. & Gallant, B. M. The intrinsic behavior of lithium fluoride in solid electrolyte interphases on lithium. Proc. Natl Acad. Sci. USA 117, 73–79 (2019).
Kim, M. S. et al. Revealing the multifunctions of Li3N in the suspension electrolyte for lithium metal batteries. ACS Nano 17, 3168–3180 (2023).
Spearman, C. The proof and measurement of association between two things. Am. J. Psychol. 15, 72–101 (1904).
Oyakhire, S. T. et al. Proximity matters: interfacial solvation dictates solid electrolyte interphase composition. Nano Lett. 23, 7524–7531 (2023).
Oyakhire, S. T. & Bent, S. F. Interfacial engineering of lithium metal anodes: what is left to uncover? Energy Adv. 3, 108–122 (2023).
Yu, Z. et al. Rational solvent molecule tuning for high-performance lithium metal battery electrolytes. Nat. Energy 7, 94–106 (2022).
Peled, E., Golodnitsky, D. & Ardel, G. Advanced model for solid electrolyte interphase electrodes in liquid and polymer electrolytes. J. Electrochem. Soc. 144, L208 (1997).
Cui, Z. et al. Molecular anchoring of free solvents for high-voltage and high-safety lithium metal batteries. Nat. Commun. 15, 2033 (2024).
Zhang, W. et al. Recovery of isolated lithium through discharged state calendar ageing. Nature 626, 306–312 (2024).
Otto, S.-K. et al. In-depth characterization of lithium-metal surfaces with XPS and ToF-SIMS: toward better understanding of the passivation layer. Chem. Mater. 33, 859–867 (2021).
Baer, D. R. XPS guide: charge neutralization and binding energy referencing for insulating samples. J. Vac. Sci. Technol. A 38, 031204 (2020).
Greczynski, G. & Hultman, L. Compromising science by ignorant instrument calibration—need to revisit half a century of published XPS data. Angew. Chem. Int. Ed. 59, 5002–5006 (2020).
Liu, Q. et al. A fluorinated cation introduces new interphasial chemistries to enable high-voltage lithium metal batteries. Nat. Commun. 14, 3678 (2023).
Ge, S. et al. High safety and cycling stability of ultrahigh energy lithium ion batteries. Cell Rep. Phys. Sci. 2, 100584 (2021).
Wood, K. N. & Teeter, G. XPS on Li-battery-related compounds: analysis of inorganic SEI phases and a methodology for charge correction. ACS Appl. Energy Mater. 1, 4493–4504 (2018).
Rustomji, C. S. et al. Liquefied gas electrolytes for electrochemical energy storage devices. Science 356, eaal4263 (2017).
Ren, X. et al. Enabling high-voltage lithium-metal batteries under practical conditions. Joule 3, 1662–1676 (2019).
Templeton, D. M. et al. Guidelines for terms related to chemical speciation and fractionation of elements. Definitions, structural aspects, and methodological approaches (IUPAC recommendations 2000). Pure Appl. Chem. 72, 1453–1470 (2009).
Feldmann, J. et al. Microwave-assisted sample preparation for element speciation. in Microwave-Assisted Sample Preparation for Trace Element Determination 281–312 (Elsevier, 2014).
Greczynski, G. & Hultman, L. Towards reliable X-ray photoelectron spectroscopy: sputter-damage effects in transition metal borides, carbides, nitrides, and oxides. Appl. Surf. Sci. 542, 148599 (2021).
First Appeared on
Source link






