Lab-grown human embryo model produces blood cells | Science
Scientists have grown embryo-like structures in the laboratory that produced human blood cells, raising new possibilities for regenerative medicine.
The ability to generate blood stem cells in the laboratory may one day make it possible to treat patients in need of bone marrow transplants using their own cells.
The advance is the latest in a rapidly advancing field in which embryo models are created from stem cells without the need for eggs or sperm, opening a window on the earliest stages of human development.
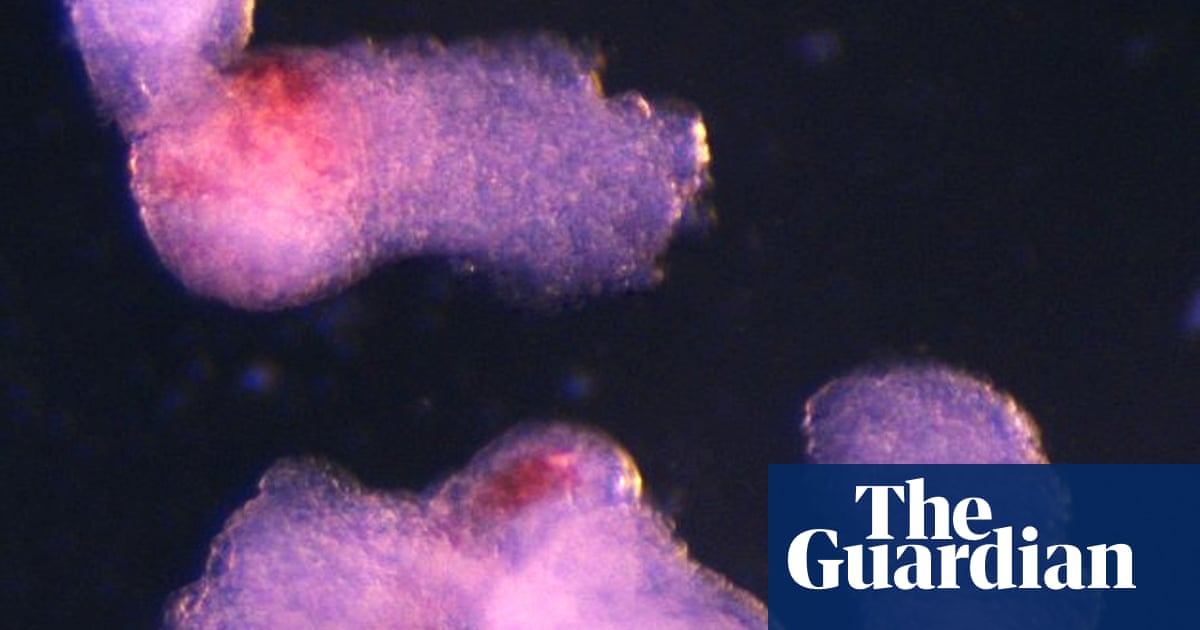
“It was an exciting moment when the blood-red colour appeared in the dish – it was visible even to the naked eye,” said Dr Jitesh Neupane, a researcher at the University of Cambridge’s Gurdon Institute and first author of the study.
He and his colleagues are using the model system to understand the earliest developmental stages of heart and blood development.
“This sheds light on how blood cells naturally form during human embryogenesis, offering potential medical advances to screen drugs, study early blood and immune development, and model blood disorders like leukaemia,” said Neupane.
The human stem cells used to grow the embryo-like structures can be created from any cell in the body. This means the approach could also pave the way for the production of blood that is fully compatible with a patient’s own body.
Although other methods exist for generating human blood stem cells in the laboratory, these require a cocktail of extra proteins, whereas the new method mimics the natural developmental process in which self-organising structures drive the formation of different cell types.
“Although it is still in the early stages, the ability to produce human blood cells in the lab marks a significant step towards future regenerative therapies – which use a patient’s own cells to repair and regenerate damaged tissues,” said Prof Azim Surani at the Gurdon Institute, senior author of the paper.
In this latest study, scientists used human stem cells to replicate some of the cells and structures that would typically appear in the third and fourth week of pregnancy. The model was specifically designed to lack the tissues that go on to form the placenta and yolk sac in a natural embryo, meaning that it did not have the theoretical potential of developing into a foetus and did not develop the tissues that would go on to form the brain.
“This is a minimalistic system,” said Neupane.
The team observed the emergence of the three-dimensional embryo-like structures under a microscope. By the second day, they had self-organised into three germ layers – called the ectoderm, mesoderm and endoderm – the foundations of the human body plan. By day eight, beating heart cells had formed, the cells that eventually give rise to the heart in a developing human embryo.
By day 13, the team saw red patches of blood appearing. Blood stem cells taken from the model were also shown to be able to differentiate into various blood cell types, including oxygen-carrying red blood cells and white blood cells that are crucial to the immune system.
The findings are published in the journal Cell Reports.
First Appeared on
Source link






