
Main Cause of Sunburn Finally Revealed, and It’s Not the Reason We’ve Always Believed
Scientific consensus on the biological mechanisms underpinning sunburn has remained largely unchanged for decades. The prevailing view posited that ultraviolet (UV) radiation induces DNA mutations in skin cells, triggering inflammatory responses and initiating cell death to prevent carcinogenesis. This framework has shaped dermatological education, cancer research funding priorities, and public health messaging across high-UV index regions.
Researchers from the University of Copenhagen and Nanyang Technological University (NTU Singapore) identified a different primary trigger. Through controlled experiments involving both murine and human epidermal models, the study demonstrated that the initial cellular response to UVB exposure is governed by RNA damage, not DNA lesions. This distinction alters the molecular understanding of cutaneous inflammation and raises new questions about the mechanisms of UV-induced cell signaling.
The findings, published in Molecular Cell, have attracted growing attention from biomedical regulatory agencies and skin disease researchers exploring alternative models of UV damage signaling. In mouse models lacking the ZAK alpha kinase gene, a key regulator in ribosomal stress detection, typical UV-induced responses such as keratinocyte death and epidermal swelling were suppressed. This suggests that ZAK alpha’s activation by damaged messenger RNA (mRNA) precedes and may override DNA-dependent pathways.
RNA Damage as the Signal Origin
The core mechanism centers on what researchers described as the ribotoxic stress response (RSR). Upon UVB exposure, damaged mRNA triggers ZAK alpha to initiate inflammatory signaling and programmed cell death. “We found that the first thing the cells respond to after being exposed to UV radiation is damage to the RNA,” said Simon Bekker-Jensen, professor of molecular medicine at the University of Copenhagen, “and that this is what triggers cell death and inflammation of the skin.”
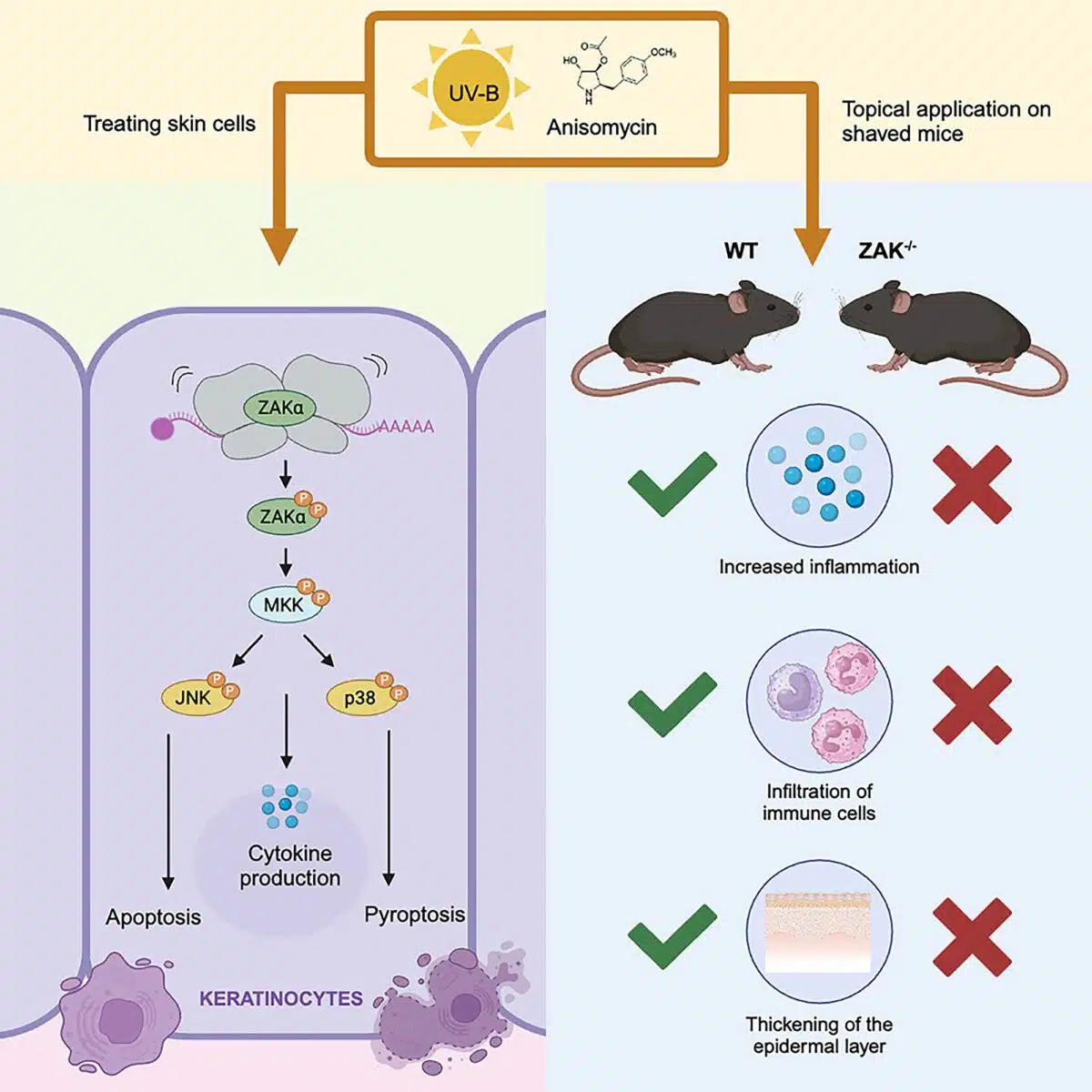
In knockout mice lacking the ZAK gene, those acute inflammatory markers were notably absent. In vitro tests using cultured human keratinocytes revealed the same pathway activation: RNA damage activated pyroptotic and apoptotic death via the p38 and JNK signaling routes, respectively. This dual-pathway activation reinforces the premise that ZAK alpha governs early UV stress responses through cytoplasmic signals rather than nuclear genome surveillance.
The Friction Point: Diagnostic, Therapeutic, and Textbook Realignment
The immediate friction point is conceptual and infrastructural. Sunscreen formulations, UV protection protocols, and dermatological interventions have historically been modeled around DNA-centric assumptions. The shift toward RNA-first mechanisms compels a reevaluation of photoimmunology models, particularly for inflammatory conditions such as polymorphic light eruption or UV-aggravated eczema.
Furthermore, ZAK alpha’s role as a molecular sentinel introduces both therapeutic targets and diagnostic markers. Potential pharmaceutical interventions that modulate the RSR pathway may offer new methods for controlling UV-induced skin inflammation, independent of mutagenic DNA repair processes. However, these applications remain theoretical; as of January 2026, no ZAK alpha inhibitors have entered clinical trials.

The research, conducted by faculty at the Department of Cellular and Molecular Medicine at the University of Copenhagen, in collaboration with NTU’s Lee Kong Chian School of Medicine, indicates a broader need for academic and medical reassessment. Assistant Professor Anna Constance Vind, a co-author of the study, noted, “This new knowledge turns things upside down. I think most people associate sunburn with DNA damage; it is established knowledge.”
Educational and clinical materials reliant on DNA-centric inflammation models will likely need revision to include the role of RNA and ribosomal stress pathways. Key areas of impact include:
- Medical curricula covering dermatology, cell signaling, and photobiology
- Textbooks and training programs for dermatologists and general practitioners
- Clinical diagnostics involving inflammatory skin responses to UV
- Drug discovery pipelines targeting early UV damage detection and response
Whether this RNA-centric model applies uniformly across skin types, UV spectrum ranges, and environmental exposure durations remains under investigation. The interaction between long-term DNA mutations and acute RNA signaling is also unresolved; they may operate on separate timelines, with independent but complementary biological effects.
First Appeared on
Source link






