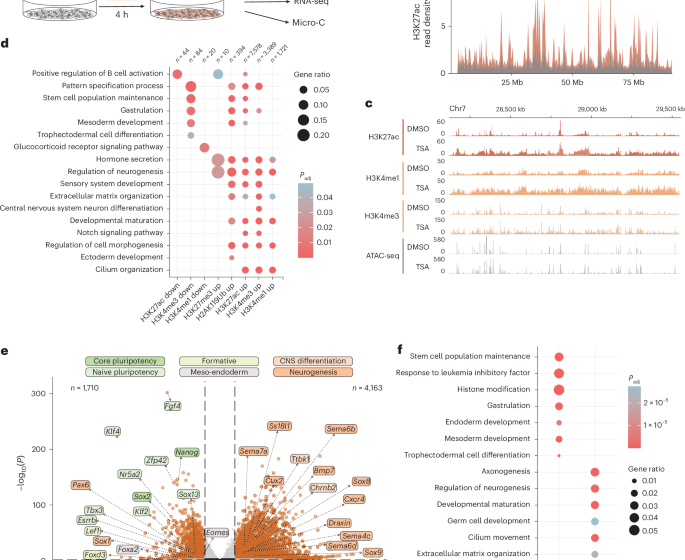
Transient histone deacetylase inhibition induces cellular memory of gene expression and 3D genome folding
ESC culture
E14GT2a p14 cells were purchased from MMRRC, UC Davis. Pcgf4−/−; Pcgf2fl/fl cells were gift from R. Klose (University of Oxford), Pcgf4 deletion and Pcgf2 excision in response to tamoxifen (OHT) were verified by genotyping PCR. CTCF–AID–eGFP cells were a gift from R. Saldana-Meyer (Howard Hughes Medical Institute); CTCF–AID–eGFP expression was confirmed by anti-GFP immunofluorescence. ESCs were cultured on plastic plates coated with 0.1% gelatin (Sigma-Aldrich, G1890-100G) in serum-LIF medium (GMEM (Gibco, 2171002), with 15% FBS (Thermo Fisher Scientific, 26140079), 1× GlutaMAX (Thermo Fisher Scientific, 35050038), 1× MEM nonessential amino acids (Thermo Fisher Scientific, 11140035), 50 U penicillin–streptomycin (Gibco, 15140122), 0.1 mM sodium pyruvate (Gibco, 11360070), 0.1 mM 2-mercaptoethanol (Gibco, 31350010) and 1,000 U ml−1 LIF (Sigma-Aldrich, ESG1107)). Cells were passaged every 2–3 days using TrypLE Express Enzyme (Gibco, 12604013). Cell lines were regularly tested for mycoplasma infection. Cell viability was assessed by staining with trypan blue (Gibco, 15250061), and cells were counted on a Countess 3 automated cell counter (Invitrogen). HDAC inhibition was performed by treating cells with 100 ng ml−1 TSA (Sigma-Aldrich, 647925) for 4 h or with 0.03 nM romidepsin for 6 h. Control cells were treated with 0.01% DMSO for the same duration. PCGF2 depletion was induced by growing Pcgf4−/−; Pcgf2fl/fl cells in medium supplemented with 800 nM 4-hydroxytamoxifen (OHT) for 72 h before each experiment. For recovery, cells were washed once with PBS and were incubated with fresh mESC medium for 10 min. This PBS wash/medium change was repeated twice before incubating cell for a total of 24 h.
Differentiation of NPCs
NPCs were grown using previously published retinoic acid-based protocol80. Briefly, 4 × 106 ESCs per replicate were cultured in suspension in Petri dishes in high glucose DMEM (Thermo Fisher Scientific, 21710025) supplemented with 1× GlutaMAX (Thermo Fisher Scientific, 35050038), 1× nonessential amino acids (Thermo Fisher Scientific, 11140035), 0.1 mM 2-mercaptoethanol (Thermo Fisher Scientific, 31350010), 1× penicillin–streptomycin (Thermo Fisher Scientific, 10378016) and 10% FBS (Thermo Fisher Scientific, 26140079). After 4 days, the medium was supplemented with 5-µM retinoic acid for an additional 4 days. The medium was changed every 2 days. Following, NPCs were replated onto gelatin-coated cell culture plates and allowed to reattach for 2 days, after which TSA treatment and washes were performed as with ESCs.
Gastruloid culture
Gastruloids for RNA-seq and immunostaining experiments were generated as described in ref. 81. Briefly, CTCF–GFP–AID cells were collected, centrifuged and washed twice with PBS. Cells were then resuspended in N2B27 medium and counted. A total of 300 cells were seeded in each well of a round-bottomed, low-attachment 96-well plate (Greiner, 650970) in N2B27 medium. After 48 h, a 24-h pulse of 3-µM CHIR99021 (Tocris Bioscience, 4423; Chiron) was administered and medium was changed every day. HDAC inhibition was carried out by treating gastruloids with 20 ng ml−1 TSA (Sigma-Aldrich, 647925) for 4 h immediately before the Chiron pulse (44–48 h). TSA was removed from the medium by changing N2B27 medium thrice with 10 min of incubation in between. Control cells were washed similarly.
Western blotting
For western blotting ~107 mESCs were dissociated, washed once in PBS, resuspended in 200 μl of cell lysis buffer (85 mM KCl; 0.5% NP40; 5 mM HEPES pH 8; 1× ethylenediaminetetraacetic acid-free protease inhibitor (Roche); 5 mM sodium butyrate) and incubated on ice for 15 min. Afterwards, nuclei were pelleted at 2000g for 5 min at 4 °C. The supernatant (cytoplasmic fraction) was separated, and nuclei were resuspended in 100-μl RIPA buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1% NP40, 0.5% NaDoc, 0.1% sodium dodecyl sulfate, 1× ethylenediaminetetraacetic acid-free protease inhibitor (Roche, 04693132001), 5 mM sodium butyrate). After a 10-min incubation on ice, chromatin was digested for 15 min at 37 °C with 0.0125 U μl−1 MNase and 1 mM CaCl2. Extracts were cleared by 30 min of centrifugation of >10000g at 4 °C. Protein yield was quantified using the Pierce BCA protein assay kit (Thermo Fisher Scientific, A65453). Samples were mixed with 4× NuPAGE LDS sample buffer (Thermo Fisher Scientific, NP0007) and boiled for 10 min at 95 °C. Furthermore, 2-µg denatured protein extract was loaded per lane on a NuPAGE 4–12%, Bis–Tris gel (Thermo Fisher Scientific, NP0321BOX). Transfer onto nitrocellulose membranes was performed using the Trans-Blot Turbo Transfer System (Bio-Rad). Membranes were stained with Ponceau S for 5 min, then blocked for at least 30 min with 3% BSA in PBS + 0.1% Triton X-100 before incubation with primary antibody overnight at 4 °C with the following dilutions: α-H3K27ac (1:7,500; Active Motif, 39133), α-pan-acetyl lysine (1:1,000; Thermo Fisher Scientific, 66289-1-IG), α-acetyl-tubulin (1:2,000; Sigma-Aldrich, T7451), H3K4me1 (1:5,000; Active Motif, 39297), H3K4me3 (1:1,000; Milipore, 04-745), H3K9me3 (1:2,000; Abcam, ab8898), H3K27me3 (1:2,500; Active Motif, 39155), H2AK119ub (1:2,000; Cell Signaling Technology, 8240S), H3K9ac (1:7,500; Millipore, 07-352) and α-lamin B1 (1:10,000; Abcam, ab16048). Membranes were washed thrice >5 min in PBS + 0.1% Tween-20 and were incubated with secondary antibodies (α-rabbit IgG–peroxidase antibody (Sigma-Aldrich, A0545) or α-mouse IgG–peroxidase antibody (Sigma-Aldrich, A9044)) at 1:16,000 dilution for 1 h at room temperature. After three >5-min washes with PBS 0.1% Tween-20 at room temperature, membranes were developed using the SuperSignal West Dura Extended Duration Substrate solution (Thermo Fisher Scientific, 34075) for 1 min and imaged with a Bio-Rad ChemiDoc imager.
Flow cytometry
A quantity of 1–3 × 106 mESCs were dissociated with TrypLE, and pelleted and resuspended in PBS. For cell cycle analysis, dissociated mESCs were washed once in PBS and pelleted and fixed in cold 70% ethanol for 30 min at 4 °C. Cells were stained with the propidium iodide flow cytometry kit (Abcam, ab139418) according to the manufacturer’s instructions. Flow cytometry was performed on a CytoFlex instrument using CytExpert (v2.4), and analysis was performed using FlowJo (v10.10). For cell proliferation tracing, dissociated mESCs were stained with 1 μM CellTrace Violet staining solution (Invitrogen, C34571) according to the manufacturer’s instructions, and were plated on gelatin-coated cell culture dishes. After 24 h, TSA treatment and washes were performed as described before and cells were collected after a further 24-h incubation period. Collected cells were fixed in 4% PFA for 10 min at room temperature, washed with PBS and preserved at 4 °C until further use. Flow cytometry was performed on a NovoCyte Quanteon, and data analysis was performed using NovoExpress (v1.6.3).
mESC immunostaining
ESCs were seeded onto glass coverslips precoated with 0.1% gelatin (Sigma-Aldrich, G1890-100G). Two hours after seeding, cells were treated with 100 ng ml−1 TSA (Sigma-Aldrich, 647925) or with 0.01% DMSO for 4 h, then rinsed with PBS and fixed with 4% paraformaldehyde (Thermo Fisher Scientific, 28906) for 10 min. After three more PBS washes, cells were permeabilized for 15 min with fresh PBS + 0.3% Triton X-100. After four washes with PBT (PBS + 0.02% Tween-20), the blocking was performed using PBT + 2% BSA for at least 30 min. Then, cells were incubated with primary antibody α-H3K27ac (1:200; Active Motif, 39133) in PBT + 2% BSA for 72 h at 4 °C with orbital shaking to prevent antibody trapping82. Afterward, cells were washed four times with PBT and stained with secondary antibody α-rabbit 555 (Invitrogen, A31572) for 1 h at room temperature. After a new round of four washes with PBS, cells were counterstained with 0.2 µg ml−1 DAPI for 10 min at room temperature on orbital shaker, before being rinsed twice with PBS. Coverslips were mounted in ~15-µl Vectashield (Eurobio Scientific, H1000) and stored at 4 °C before imaging.
Image acquisition and quantification
DMSO and TSA conditions in each experiment were imaged and analyzed using the same parameters. Confocal imaging was performed using a Zeiss confocal LSM980 Airyscan 2 equipped with ×63 (ESCs) or ×20 (gastruloids) objectives using ZEN Blue (v3.8-3.12). Diodes laser 405, 488, 561 and 639 nm were used for fluorophore excitations. For each gastruloid, three z stacks were taken and, using Fiji (v2.14.0), maximum intensities were projected to manually define areas of H3K27ac, Sox2 and Brachyury expression and DAPI staining. For ESCs, several z stacks were taken for each condition. Quantification of nucleus volumes and H3K27ac distances to periphery was performed using Imaris (v10.1.1). The option ‘surfaces’ was used to segment nuclei, H3K27ac signal was analyzed as ‘spots’ (xy diameter = 0.3 µm/z diameter = 0.6 µm). ‘Distance transformation’ was used to generate distance to periphery, and the option ‘split spots into surface objects’ was used to assign spots to the corresponding nuclei. The distance between each spot and the periphery is given by the intensity of the ‘distance transformation’ channel at the center of each spot.
Gastruloid immunostaining
Gastruloid immunostaining protocol was adopted from ref. 83. Plastic material was precoated with blocking solution (PBS + 10% FBS + 0.2% Triton X-100). Using a cut P1000 tip, gastruloids were collected into 15-ml centrifuge tubes. After a PBS wash, gastruloids were transferred to 2 ml of 4% PFA in six-well plates and fixed overnight at 4 °C. For washes, gastruloids were transferred serially across three PBS-filled wells and were incubated for 10 min in the last one. Gastruloids were blocked in PBS + FT (PBS + 10% FBS + 0.2% Triton X-100) for 1 h at room temperature, then incubated with primary antibodies (α-brachyury (1:500; Santa Cruz Biotechnologies, sc-166962), α-Sox2 (1:500; eBioscience, 15208187), α-H3K27ac (1:200; Active Motif, 39133)) in PBS + FT and 1 µg ml−1 DAPI overnight at 4 °C with orbital shaking. Gastruloids were washed by sequentially transferring them to three wells filled with PBS + FT and incubating them for 20 min in the last one. Staining with secondary antibody (α-rabbit Alexa Fluor Plus 488 (1:400; Thermo Fisher Scientific, A32731), α-rabbit Alexa Fluor Plus 555 (1:400; Thermo Fisher Scientific, A32794), α-rabbit Alexa Fluor Plus 647 (1:400; Thermo Fisher Scientific, A48265)), and 1 µg ml−1 DAPI, as well as washes were carried out similarly to primary antibody. Gastruloids were mounted in ~30-µl Fluoromount-G (Thermo Fisher Scientific, 00-4958-02) and were kept at 4 °C before imaging.
RNA isolation for RNA-seq
RNA was isolated using the RNeasy mini kit (Qiagen, 74104). Cells were detached with TrypLE, lysed in RLT buffer with β-mercaptoethanol and lysates were processed according to the manufacturer’s instructions. For mESCs columns and buffers supplied with the RNeasy kit were used, while for gastruloids, the Zymo RNA Clean & Concentrator-5 (Zymo Research, R1015) reagents were used. On-column DNase-I digestion (Qiagen, 79254) was performed as recommended. RNA samples were sent to BGI Tech Solutions for strand-specific transcriptome sequencing. Samples were sequenced at a depth of 50 million 150-bp paired-end reads.
Micro-C library preparation and sequencing
Micro-C libraries were generated with the Dovetail Micro-C Kit protocol (v1.0) with minor modifications. Briefly, 106 mESCs were washed with PBS and were frozen at −80 °C for at least 1 h. Cell pellets were thawed and crosslinked first with 3 mM DSG (Thermo Fisher Scientific, A35392) in PBS for 10 min at room temperature with rotation, then formaldehyde was added at 1% final concentration for a further 10 min. The pellets were washed twice with PBS and digested with MNase according to the kit instructions. MNase digestion was routinely verified by decrosslinking a small amount of chromatin and assessing fragment distribution on a Bioanalyzer 2100 instrument (Agilent). If the digestion profile showed 50–70% mononucleosomal DNA fraction, on-bead proximity ligation was performed, followed by crosslink reversal and DNA purification. End repair and adaptor ligation were performed using the NEBNext Ultra II DNA Library Prep Kit for Illumina (NEB, E7645). Following, DNA was purified using Solid Phase Reversible Immobilization beads (Beckman, B23318) as described in the Micro-C user manual. Finally, biotin pulldown and library amplification were performed according to the Dovetail Micro-C Kit User Guide and using Dovetail Micro-C Kit reagents, only replacing the Dovetail Primers (Universal and Index) with NEBNext primers. Libraries were pooled and sent to BGI Tech Solutions for 100-bp paired-end sequencing to obtain roughly 2–3 billion reads per replicate.
ChIP
ChIP was performed as described previously84. Cells were collected with TrypLE (Thermo Fisher Scientific, 12604013) and fixed in mESC medium containing 1% methanol-free formaldehyde for 10 min with rotation at room temperature. Glycine (2.5 M glycine in PBS) was used to stop the fixation for 10 min with rotation at room temperature. Fixed cells were centrifuged at 500g for 5 min at 4 °C, washed twice in 1× ice-cold PBS and snap frozen in liquid nitrogen until further use. After thawing, cells were spiked-in with 8% HEK-293 cells and chromatin extraction was performed as discussed in ref. 85 with sonication on a Covaris E220 instrument (Duty Factor 5%; PIP 140 W; cycles per Burst 200; 12 min). A total of 15 μg of chromatin was used for each replicate of histone ChIP, and 50 µg for CTCF, YY1 and Ring1B ChIP, with 6–8 μg of antibody. Because the above protocol was not suitable for YY1, we followed the protocol described in ref. 86. Briefly, fixed cells were resuspended in sodium dodecyl sulfate buffer, followed by sonication and preparation for immunoprecipitation. Next, the mixture was incubated overnight at 4 °C with Protein G beads (Invitrogen, 10004D), washed with both low and high-salt buffers, reverse-crosslinked in elution buffer, and purified using a QIAQuick PCR purification kit (Qiagen, 28104). Antibodies used in this study were as follows: H3K4me1 (Active Motif, 39297), H3K4me3 (Milipore, 04-745), H3K27ac (Active Motif, 39133), H3K9me3 (Abcam, ab8898), H3K27me3 (Active Motif, 39155), H2AK119ub (Cell Signaling Technology, 8240S), H3K9ac (Millipore, 07-352), Ring1B (Cell Signaling Technology, 5694), CTCF (Active Motif, 61311) and YY1 (Abcam, 109237). For ChIP–qPCR, the LightCycler 480 SYBR Green Master (Roche, 04887352001) was used on undiluted ChIP DNA and input DNA in 1:10 dilution. Primer sequences are provided in the Supplementary Methods. For ChIP–seq, sequencing libraries were constructed using NEBNext Ultra II DNA Library Prep Kit for Illumina (NEB, E7645), pooled and sent to BGI Tech Solutions for 100-bp paired-end sequencing to obtain roughly 30–50 million reads per replicate.
Assay for transposase-accessible chromatin using sequencing (ATAC–seq)
For each replicate, 9 × 104 mESCs were collected with TrypLE (Thermo Fisher Scientific, 12604013) and were mixed with 104 HEK-293 cells. Samples were processed using the Active Motif ATAC–seq kit (Active Motif, 53150) following the manufacturer’s instructions without modifications. ATAC–seq libraries were pooled and sent to BGI Tech Solutions for 100-bp paired-end sequencing, yielding approximately 30–50 million reads per replicate.
Statistics and reproducibility
RNA-seq experiments were performed in biological triplicates. ChIP–qPCR, ChIP–seq and ATAC–seq experiments were performed in biological duplicates, except for H3K27ac ChIP–seq in DMSO, TSA and recovery where three independent replicates were performed. Micro-C was performed in biological duplicates, except for DMSO and TSA, where five biological replicates were produced. Gastruloid immunostaining was performed in biological triplicates, mESC immunostaining in duplicates. Sample sizes are indicated in figures and/or legends. Cell viability and cell cycle profiling were performed in biological triplicates, and cell generation tracing was performed in duplicates. H3K27ac western blotting was routinely performed to verify the effects of TSA and washes. All other western blots were performed in biological duplicates, except for H3K27ac in NPCs, where a single replicate was performed.
Data collection and analyses were not performed blindly to the conditions of the experiments. No data were excluded from the analyses, except for gastruloid immunofluorescence, where gastruloids with clear morphological and/or symmetry aberrations were not imaged. The experiments were not randomized. For statistical analyses, normality was assessed using the Shapiro–Wilk test. For small sample sizes (<10), the data were assumed to be normally distributed, although this was not formally tested. No statistical method was used to predetermine sample size.
RNA-seq analysis
RNA-seq samples were mapped using the ‘align’ function of the Subread package (v2.0.6). Subread command ‘featureCounts’ (with options ‘-p –countReadPairs -s 2 -t exon’), and the feature file UCSC RefSeq GTF file for mm10 were used to generate count tables that were then used as inputs for DEseq2 (v1.42.1)87 to perform differential analysis (Supplementary Tables 1–4). GO analysis was performed using the ‘enrichGO’ function from the clusterProfiler (v4.10.1) package88 (Supplementary Table 5). Volcano plots and scatterplots were produced in R using the EnhancedVolcano (v1.20.0) and ggplot2 (v3.5.1) libraries, respectively. Motif enrichment analysis at differentially expressed genes was carried out using the ‘findMotifs.pl’ function of the HOMER (v4.10.0) Motif Discovery and Analysis tool89 (Supplementary Table 6).
ChIP–seq and ATAC–seq analysis
ChIP–seq and ATAC–seq samples were mapped using bowtie2 (v2.4.4)90 with command ‘bowtie2 -p 12 –no-mixed–no-discordant’ against the mm10 and hg19 genomes. Then, samtools (v1.9)91 was used to filter out low-quality reads (command ‘samtools view -b -q 30’) and sambamba (v1.0)92 was used to sort (command ‘sambamba sort’), deduplicate and index BAM files (‘sambamba markdup –remove-duplicates’) with default parameters. Following, samtools was used to count both human and mouse reads (command ‘samtools view -c’) to calculate the downsampling factor (dF) for spike-in normalization as described in ref. 39. Next, BAM files were downscaled accordingly using samtools (command ‘samtools view -b -s dF’) and bigwig files were produced using the deepTools package93 with command ‘bamCoverage –normalizeUsing none –ignoreDuplicates -e 0 -bs 10’. Finally, ChIP–seq tracks were visualized using IGV (v.2.16.1)94 or HiGlass (v1.11.8)95. ATAC–seq and ChIP–seq peaks were called on each replicate using MACS3 (v3.0.3) with a q-value cutoff of 0.05, and for histone marks with the additional parameters ‘–broad –broad-cutoff 0.1’ (ref. 96). Finally, peaks detected from both replicates were filtered and all downstream analyses were carried out using this consensus peak set. For differential peak calling the diffBind (v3.12.0)97, R package was used with normalization ‘normalize = DBA_NORM_LIB, spikein = TRUE’, analysis method ‘method = DBA_DESEQ2’, and false discovery rate of <0.05 cutoff (Supplementary Table 7). Heatmaps and metaplots were produced using the ‘computeMatrix’ function of the deepTools (v3.5.6) package, and plotted using the ‘plotHeatmap’ and ‘plotProfile’ functions. ChIP–seq box plots were also created by deepTools using the ‘multiBigwigSummary’ function and were plotted by ggplot2 (v3.5.1) in R. Chromosome-wide H3K27ac read density plots were generated using a custom R script published in ref. 39. ChIP–seq peak distribution and annotation were carried out with ChIPseeker’s98 (v1.38.0) ‘plotPeakProf’ and ‘annotatePeak’ functions, respectively. GO analysis of annotated ChIP–seq peaks was performed using the ‘enrichGO’ function from the clusterProfiler (v4.10.1) package88 (Supplementary Table 5). Differential ATAC–seq peaks were analyzed with the i-cisTarget online tool99,100, using v.6.0 of the position weight matrix database filtered for hits in the HOMER database (Supplementary Table 6). For cumulative histograms, enhancer distance from TSSs was calculated using bedtools (v2.31.1) ‘closest’ function and was plotted by ggplot2 (v3.5.1) in R. Expression-matched control gene set was derived using code from the AdelmanLab github repository (https://github.com/AdelmanLab/Expression-Matching). Myc ChIP–seq in ESC and H3K27me3 in NPCs were published previously101,102.
Micro-C data analysis
Generation of contact matrices and standard analyses
Micro-C data were mapped using the HiC–Pro (v3.1.0) pipeline103. FASTQ reads were trimmed to 50 bp using TrimGalore (v0.6.10; ‘–hardtrim5 50’; https://github.com/FelixKrueger/TrimGalore) and aligned to the mm10 reference genome using bowtie2 (ref. 90; v2.4.4; ‘–very-sensitive –L 30 –score-min L, -0.6, -0.2 –end-to-end –reorder’), removing singleton, multihit and duplicated reads. Minimum cis-distance was set at 200 bp. The total numbers of valid read pairs per sample are reported in Supplementary Table 8. Contact matrices in the .cool file format were generated using cooler104 (v.0.10.2) at 100-bp resolution (command ‘cooler cload pairs -c1 2 -p1 3 -c2 5 -p2 6./scripts/chrom_sizes.txt:100’). Similarities between replicates (five replicates for DMSO and TSA; two replicates for 24-h recovery) were measured applying ‘HiCRep’ (v1.12; https://github.com/TaoYang-dev/hicrep)105 on chromosomes 2, 9, 13 and 19 using the ‘get.scc’ function with parameters resol = 20 kb and (lbr,ubr,h) = ((0, 100 kb,1), (100 kb, 500 kb,1), (500 kb, 2 Mb,2), (2 Mb, 10 Mb,4)). H values were previously trained using the ‘htrain()’ on two replicates of the DMSO condition. Using 1.0-SCC, as a measure of the similarity (0 = similar and 1 = dissimilar) between replicates and hierarchical clustering analysis using ‘hclust()’ function in R with Ward.D2 method on the chromosome-averaged similarities, allowed to distinguish and group together the replicates of the different conditions, motivating to merge the valid-pairs of different replicates in a unique dataset for each condition. Multiresolution ‘.mcool’ files were obtained and normalized through the Iterative Correction and Eigenvector decomposition algorithm (ICE) with default parameters (command ‘cooler zoomify -r resolutions file.cool -o file.mcool –balance’)106 and were uploaded onto a local HiGlass (v1.11.8) server for visualization95. For comparison of architectural features among different conditions, contact maps were matched to contain approximately the same number of cis-contacts (Supplementary Table 8). All genomic snapshots of Micro-C maps were generated using HiGlass (v.1.11.8). Standard analyses (cis-decay curves, eigenvector decomposition, saddle plots) were performed using the cooltools (v0.5.4) package.
Loop analyses
Loops were called using mustache (v1.0)107 with default parameters (‘–pThreshold 0.1 –sparsityThreshold 0.88 –octaves 2’) on ICE-balanced maps at 1-kb and 4-kb resolutions. Redundant loops among different resolutions were filtered in 20-kb windows, and coordinates were retained at the finer resolution. All aggregate plots were created with the coolpuppy (v1.1.0) package108 and were normalized using expected maps generated by cooltools (v0.5.4). For differential looping, contacts that overlapped with the corresponding loop anchor bin were summed for each loop and were summarized into a count table—genome-wide count tables were created for each replicate at each resolution (command ‘cooler dump –join -t pixels’), then filtered against loop using bedtools (v2.31.1) ‘pairtopair’ function109. The count tables from different conditions were used for differential analysis with DESeq2 (v1.42.1; Supplementary Tables 9 and 10). The thresholds Padj < 0.05, |log2 fold change (FC)| > 0.5 and baseMean ≥ 10 were used to filter for substantial changes in looping between conditions. Volcano plots were produced in R using the EnhancedVolcano (v1.20.0) library. Loop subclasses were defined based on the presence of ChIP–seq peaks at loop anchors (repressive—overlapping with H3K9me3, H3K27me3 or H2AK119ub peaks; active—overlapping with H3K4me1, H3K4me3 or H3K27ac peaks; de novo H3K9me3 loops—loops only present in TSA overlapping with H3K9me3 peak; CTCF—loop anchors within ±1 kb of CTCF peaks; non-CTCF—no CTCF peak within ±2.5 kb of loop anchor) or the presence of TSSs within 2 kb of either loop anchor (Supplementary Table 9). E–P contacts for recovery versus nonrecovery genes were taken from ref. 20. Loop quantification box plots represent the observed/expected value of the central 3 × 3 pixels of aggregate plots that was extracted from coolpuppy matrices using an in-house Python script. Loop anchors were annotated using the ‘annotatePeak’ function of the ChIPseeker (v1.38.0) R package, and annotated anchors within <10 kb from TSSs were used for GO enrichment with the ‘enrichGO’ function of clusterProfiler (v4.10.1) library (Supplementary Table 5).
Biophysical modeling
Biophysical modeling was performed as described in the Supplementary Methods.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
First Appeared on
Source link






